Medicine & Health
High-end chemical drug Preparations Industrial Park Infrastructure Construction Project of Tonghua City
1 Introduction to the Project
1.1 Project background
1.1.1 Product introduction
High-end drug preparations are improved and innovated on the basis of traditional preparations, with the primary goal of overcoming treatment deficiencies and achieving clinical advantages. By changing the physicochemical properties and metabolic characteristics of the drugs, they can improve the treatment efficacy, reduce toxic side effects, improve medication compliance, meet clinical needs, and benefit patients more.
In the context of normalized centralized procurement of drugs in China, the medicine industry has developed into the second half of high-end upgrading and differentiation breakthrough. Moreover, the investment and risk of innovative drug development are high, and some targets face severe homogenization competition. Meanwhile, high-tech and high-barrier high-end preparations can be a “shortcut” for innovation. Compared to ordinary generic drugs, high-end preparations have higher research and development (R&D) processes and market access barriers, with fewer competitors; Compared to innovative drugs, high-end preparations have a shorter R&D cycle, which can effectively save the R&D costs.
Making use of the good foundation of the medicine industry development in Tonghua City, the project constructs the infrastructure project of the high-end biochemical drug preparation industrial park in Tonghua City. It plans to introduce 30-50 biopharmaceutical R&D and production enterprises to create an important driving force for the development of the regional medicine and health industries.
1.1.2 Market prospect
(1) The current situation of medicine industry in China
The medicine industry is an important component of China’s national economy, combining traditional and modern industries, and integrating the three industries (primary, secondary and tertiary industries). With the improvement of the living standards of the domestic people and the increasing demand for health care, the medicine industry is receiving more and more attention from the public and the government, and occupies an increasingly important position in the national economy.
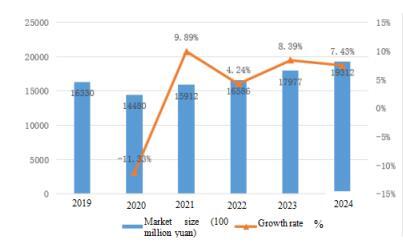
Thanks to the improvement of people’s living standards, the increase in medical demand, and the continuous progress of medicine technology, especially with the intensification of population aging, the rigid demand for medical devices and drugs continues to grow, injecting new vitality into the medicine market. In addition, the incidence rate of cardiovascular diseases, diabetes, cancer and other chronic diseases has increased year by year, promoting the demand for related drugs and treatment methods. With the increasing awareness of health among people, the demand for preventive medicine and health management is also constantly growing. The overall market size of medicine in China is showing a growth trend, with a market size of 1.7977 trillion yuan in 2023, a year-on-year growth of 8.39%. The market size of medicine in China reached 1.9312 trillion yuan in 2024.
Table 1 Market Size and Growth Rate of Medicine Industry in China from 2019 to 2024
(2) The current situation of medicine manufacturing industry in China
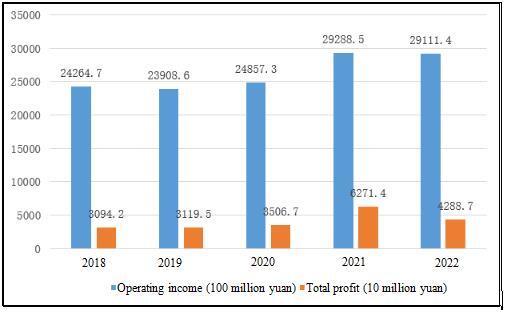
Affected by centralized procurement and medical insurance negotiations, the income and profit of the medicine manufacturing industry have declined since 2022. The In-depth Analysis of the Medicine Manufacturing Industry in China from 2024 to 2029 and Research Report on Development Opportunities during the 14th Five-year Plan released by the ASKCI Industry Research Institute showed that, in 2022, the operating income of the enterprises above designated size of the medicine manufacturing industry in China was 2.91114 trillion yuan, a year-on-year decrease of 1.6%, and the total profit was 428.87 billion yuan, a year-on-year decrease of 31.8%.
Table 2 Operating Income and Total Profit of the Enterprises above Designated Size of the Medicine Manufacturing Industry in China from 2018 to 2022
(3) Market analysis of high-end chemical drug preparations
With the aging population and increasing health awareness, the people’s demand for health care is constantly increasing. The efficacy and high-quality characteristics of high-end preparations have made them increasingly favored by the doctors and the patients. In addition, with the progress of medical technology, the variety and quantity of high-end preparations will continue to increase.
At present, the production level of preparations in China still lags behind the international level. Chemical preparations are mainly generic drugs, and the proportion of innovative preparations is relatively low. Innovative preparations developed domestically have not been internationally recognized and cannot enter the international market. Developed countries import intermediates and active pharmaceutical ingredients (APIs) from China to prepare high-end preparations, and then use their advantages in original drugs and brands to enter China and seize the market, which has had a strong impact on China’s medicine industry. In the field of preparation research and development, high-end preparations have the advantages of low development investment, short cycle, and low clinical risks compared to new molecular entities. They can not only create heavyweight products, but are also difficult to imitate and can occupy the market for a long time. Compared with developed countries, China’s medicine industry has long been in a deformed development model of “focusing on APIs and making light of preparations”, resulting in rapid development of APIs and lagging development of preparations. The added value of the products is generally low, with poor competitiveness, leading to low-level imitation of ordinary dosage forms and the dilemma of being unable to pass consistency evaluation. Although cutting-edge technology research and exploration are exceptionally active, the field of high-end preparation research mostly remains at the level of laboratories, papers, or patents, lacking fine and systematic in-depth researches, with low technological maturity, poor applicability and compatibility, difficulty in transforming into products, inability to achieve large scale, industrialization, and internationalization, and lack of independent innovative high-end preparation products with international influence.
The R&D and production level of high-end preparations holds an important position both domestically and internationally. High-end preparation technology has become an important breakthrough point for domestic medicine innovation, and the policies support the development of high-end preparation technology. Early in 2016, the development of high-end preparations was included in the national development plan during the “13th Five-year Plan” period; The subsequent new drug classification included innovative preparations as Class II innovative drugs; In January 2022, nine departments including the Ministry of Industry and Information Technology jointly released the 14th Five-year Plan for the Development of the Medicine Industry, which proposed to implement the medicine industrialization technology breakthrough projects. Improving the level of China’s preparation industry, enhancing the quality of preparation products, and developing high-end preparations are serious issues that the healthy development of China’s preparation industry is confronted with.
At present, many domestic pharmaceutical enterprises, including Qilu Pharmaceutical and Oryza Pharmaceuticals, are continuously increasing their R&D investment in the field of new high-end preparations, and have gradually built a series of preparation R&D and production platforms, constantly breaking through “bottleneck” technologies and achieving rapid transformation of scientific research achievements. However, for a long time, there has been a situation of “focusing on APIs and making light of preparations” in China. Currently, the domestic preparation industry is still in its infancy, and most of the high-end preparation technologies and products, including cancer drugs, orphan drugs, and drugs for children, etc., still rely on imports.
With innovation becoming the main theme of the development of the medicine industry and the increasing number of enterprises entering the field of high-end preparation technology innovation, the industry believes that high-end preparation technology will be an important breakthrough point for China’s medicine innovation and one of the important directions for local pharmaceutical enterprises to participate in global innovation.
The High-end Chemical Drug Preparations Industrial Park Infrastructure Construction Project of Tonghua City aims to construct a high-quality chemical drug product preparation production park, strengthen the construction capacity of infrastructure and public service system, introduce biopharmaceutical research and development production enterprises, continuously accelerate the landing of major varieties and projects, and promote industrial agglomeration development, with a broad prospect.
1.1.3 Advantageous conditions of project construction
(1) Policy advantages
In January 2022, nine departments including the Ministry of Industry and Information Technology jointly released the 14th Five-year Plan for the Development of the medicine industry, which clearly proposed that we should strengthen breakthrough of key core technologies, improve the level of industrialization technology, support the enterprises to integrate the scientific and technological resources, and implement medicine industrialization technology breakthrough projects. Regarding chemical drug technology, we should focus on fields such as complex preparation technologies characterized by high selectivity and long-term controlled release, etc., including injections such as microspheres, oral preparations such as sustained or controlled release and multi-particle systems, the transdermal, implantable, inhalation, and oral dissolving film drug delivery systems, and drug-device combination products, etc. Regarding biopharmaceutical technology, we should focus on fields such as ultra large-scale cell culture technology, new drug delivery methods for biopharmaceuticals, and novel delivery technologies, etc.
The Outline of the 14th Five-Year Plan for National Economic and Social Development and Vision 2035 of Jilin Province proposed to cultivate and develop high-end chemical drug preparations and establish chemical API export bases. Build medicine characteristic industrial parks in Liaoyuan, Tonghua, Baishan, Meihekou, and Dunhua, etc., and establish a national production base for small variety drugs in Northern China. By 2025, the graphene market size will have reached 200 billion yuan.
In order to support the better undertaking of industrial transfer in the Northeast China, promote the adjustment and optimization of industrial structure, and help achieve new breakthroughs in the comprehensive revitalization of Northeast China, in February 2023, the National Development and Reform Commission replied and agreed to establish a Southwest Jilin Industrial Transfer Demonstration Zone, covering three cities of Siping, Liaoyuan, and Tonghua. The demonstration zone will undertake seven major industries including medicine and health, equipment manufacturing, agricultural products and food processing, green energy carrier, modern chemical engineering, modern textiles, and cultural tourism. The project is located in the Southwest Jilin Industrial Transfer Demonstration Zone, and belongs to the medicine and health project among the seven major undertaking industries.
Tonghua Medicine High-tech Zone has excellent investment environment. It has successively introduced 35 supporting policies covering innovation environment, service system, science and technology finance, talent introduction, and high-tech enterprise development have been successively introduced, including the Implementation Opinions on Accelerating Enterprise Investment and Development, etc., and has set up a medicine industry Investment Guidance Fund with a total amount of 250 million yuan. The Several Policies for Investment Promotion and Talent Attraction in Tonghua Medicine High-tech Zone proposed to use the Medicine and Health Science and Technology Innovation Park in the Western District to build a Health Food Industrial Park. The Detailed Rules for the Settlement of Medicine and Health Science and Technology Innovation Park and Health Food Industrial Park have been issued, and the Several Policies on Encouraging Technological Innovation and Promoting High-quality Development of Enterprises have been studied and formulated. A special fund of 10 million yuan has been set up to provide financial and other support for scientific and technological research and development of the enterprises.
(2) Resource advantages
Tonghua, located at the southwest foot of Changbai Mountain, is rich in medicinal resources, enjoying the reputation of “Three Natural Medicine Banks”, “Hometown of Ginseng in China” and the “Main Origin of Genuine Medicinal Materials in Changbai Mountain”.
The preserved area of ginseng in Tonghua City is 55,000 mu, and the under-forest ginseng has been optimized to 360,000 mu, with output of fresh ginseng at 13,000 tons/year. Tonghua City cultivates more than 20 genuine small and medium-sized medicinal herbs such as Schisandra chinensis, Fritillaria cirrhosa, Platycodon grandiflorus, Asarum heterotropoides, Gastrodia elata, and Gentiana cruciata all year round, with a planting area of 64,300 mu and an output of 25,000 tons/year.
(3) Industrial advantages
Medicine industry is one of the pillar industries in Tonghua City, with a relatively strong industrial foundation and good prospects for transformation. Tonghua City, as the famous “Medical City in China” and “Ginseng City in China”, currently has 104 medical enterprises, holding 3,582 approval numbers and 153 exclusive varieties. Among them, there are 2,274 TCM approval numbers, with 875 varieties produced all year round; 1,286 chemical drug approval numbers, with 108 varieties produced all year round; 19 biomedical approval numbers, with 12 varieties produced all year round; 2 auxiliary material approval numbers; There are 695 varieties included in basic drug catalog, including 391 TCM approval numbers, 8 biological drug approval numbers, and 296 chemical drug approval numbers; 10 approval numbers for protected TCM varieties; There are 1,321 varieties included in national medical insurance catalog, including 720 TCM approval numbers, 555 chemical drug approval numbers, and 16 biological drug approval numbers. The number of approved TCM ranks first in the country.
(4) Location advantages
Tonghua has a unique location and huge opening up space. Located in the center of the Northeast Asian Economic Circle and the core area of the Yalu River International Economic Cooperation Belt, the border line is 203.5 kilometers long. It is an important link belt in the easternmost part of China to promote the “Belt and Road” strategy and achieve land sea connectivity and interaction, an important hub of the East Corridor in Northeast China, an important window for Jilin Province to open up to the south, and has one national port for highways and railways, respectively; There are 7 highways passing by Tonghua; with 467 kilometers of railway operation; and flight access from Tonghua to Beijing, Shanghai, Guangzhou, Tianjin, Chongqing, Taizhou, Dalian and other cities. The construction of Shenyang-Baishan Passenger Dedicated Line and Ji’an-Huanren Expressway, and preliminary work for expansion of Tonghua Airport are being accelerated.
(5) Talent advantages
At present, Tonghua City has 3 national key secondary vocational schools, 3 provincial key secondary vocational schools, and all its county-level vocational education centers have passed the provincial key school acceptance and entered the top 100 schools in the province. It is the first region in the province where all county-level vocational education centers have entered the provincial key school list. In recent years, by making scientific layout, continuous promotion of vocational education structure adjustment and resource integration, exploration of new paths for diversified education, and continuous improvement of modern vocational education network system construction, the local characteristics of vocational education have begun to form, and the ability to serve economic and social development has gradually improved.
Tonghua Municipal Government has signed science and technology strategic cooperation agreements with provincial universities and research institutions such as Northeast Normal University, Jilin Agricultural University, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and Tonghua Normal University, etc., providing scientific and technological support for development of the enterprises. Tonghua City continues to provide assistance for enterprises in talent cultivation and other aspects, vigorously implements the “Plan for Revitalizing Enterprises with Ten Thousand Students” and “Plan for Homing of a Thousand Students”, and continuously attracts college graduates for enterprises.
1.2 Contents and scale of project construction
The total area of the project is 130,000 square meters, with a total construction area of 160,000 square meters. It is planned to construct 12 production plants that conform to GMP of chemical drug preparations, 1 small-scale plant, 1 pilot plant, 1 R&D quality inspection center, and 1 comprehensive service center, as well as supporting infrastructure such as canteens, dormitories, fire protection, environmental protection, sewage treatment, and hazardous chemical warehouses, etc.
1.3 Total investment of the project and capital raising
1.3.1 Total investment of the project
The total investment of the project is 500 million yuan, including the construction investment of 490 million yuan and current funds of 10 million yuan.
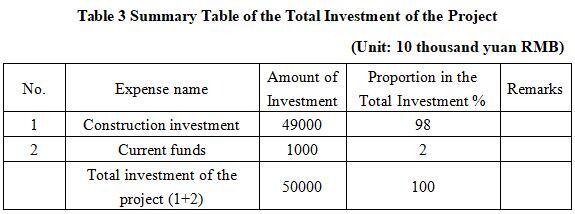
1.3.2 Capital raising
Raised by the enterprise itself.
1.4 Financial analysis and social evaluation
1.4.1 Main financial indexes
After the project reaches the production capacity, its annual sales income will be 120 million yuan, its profit will be 80 million yuan, its investment payback period will be 8.3 years (after the tax, including the construction period of 2 years), and its investment profit rate will be 16.0%.
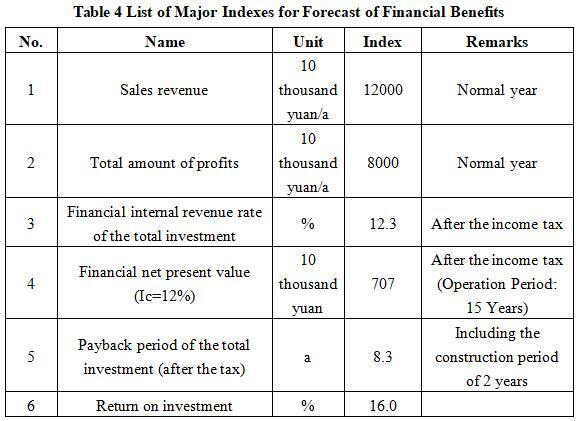
Note: “10 thousand yuan” in the table is in RMB
1.4.2 Social evaluation
Through construction of the project, the carrying capacity of the chemical drug preparation industry in Tonghua City can be improved and formed, which is of great strategic significance for reducing production costs of medicine enterprises, improving utilization efficiency of resources, creating a favorable development environment, and enhancing the agglomeration capacity of the medicine industry base. The project has good social benefits.
1.5 Cooperative way
Joint venture or cooperation, and other ways can be discussed in person.
1.6 What to be invested by the foreign party
Funds and others.
1.7 Construction site of the project
Tonghua Medicine High-tech Industrial Development Zone.
1.8 Progress of the project
The project proposal has been prepared.
2 Introduction to the Partner
2.1 Basic information
Name: Commerce Bureau of Tonghua Medicine High-tech Industrial Development Zone
Address: No. 1568 Ring Road of the Economic Development Zone, Tonghua City
2.2 Overview
The predecessor of Tonghua Medicine High-tech Industrial Development Zone (hereinafter referred to as “Tonghua Medicine High-tech Zone”) was Jilin Tonghua Economic Development Zone, and it was initially established in July 2005. In August 2011, with the approval of the Jilin Provincial People’s Government, Jilin Tonghua Economic Development Zone was integrated with Tonghua Medicine High-tech Industrial Development Zone, and was renamed as Tonghua Medicine High-tech Industrial Development Zone. In December 2013, it was promoted to be a National-level Medicine High-tech Zone with the approval of the State Council. Tonghua Medicine High-tech Zone has an approved planned area of 12.7082 square kilometers, with an administrative division area of 57.3 square kilometers; its mid-and-long-term planned area is 57 square kilometers, with an administrative division area of 107 square kilometers.
There are currently 43 medicine enterprises in Tonghua Medicine High-tech Zone, of which 7 enterprises including Wantong Pharmacy and Xiuzheng Pharmaceutical have developed into group companies, Tonghua Dongbao and Tonghua Golden-Horse Pharmaceutical are listed companies, 9 medicine enterprises have been re-recognized as national-level high-tech enterprises, and 9 enterprises have been certified as provincial-level enterprise technology centers, of which 3 enterprises have been recognized as national-level technology centers. With the implementation of “big transportation” strategy in Tonghua, the successive completion of the “six ways, one airport, and one port” projects including Tonghua-Shenyang, Tonghua-Dandong, Tonghua-Changchun, Tonghua-Ji’an and Meihekou-Shenyang Expressways, Tonghua-Dandong Railway, Tonghua Airport, and Tonghua Land Port, etc., will directly integrate Tonghua into the “2-hour economic circle” around the Bohai Sea. In particular, the implementation of the “Tonghua-Dandong Economic Belt” in the Yalu River Economic Cooperation Pilot Zone of Liaoning and Jilin Provinces, will make Tonghua an important connecting belt for the Liaoning Coastal Economic Belt, Shenyang Economic Zone, and Changchun-Jilin-Tumenjiang Development and Opening-up Pilot Zone. Tonghua Medicine High-tech Zone has a strong late-mover advantage.
2.3 Contact method
2.3.1 Contact method of cooperator
Contact unit: Commerce Bureau of Tonghua Medicine High-tech Industrial Development Zone
Contact person: Chen Kuihong, Cui Hong
Tel: +86-435-3265515
+86-13944545515
+86-435-3322595
+86-13732858668
E-mail: thyygxq@126.com
2.3.2 Contact method of the city (prefecture) where the project is located
Contact unit: Tonghua Municipal Commerce Bureau
Contact person: Wang Liangchen
Tel: +86-435-3199017
+86-18643036783
E-mail: thsswjtck@126.com


